Bacteria in fungi provide clues to the origins of complex life
Scientists implant bacteria into fungi to decipher the origins of complex life and create new symbioses.
Bacteria in fungi provide clues to the origins of complex life
Scientists using a tiny hollow needle and a bicycle pump have managed to implant bacteria into a larger cell. This creates a relationship similar to those that drove the evolution of complex life.
This achievement, published October 2 in the journal Nature 1, could help researchers understand the origins of partnerships that led to the emergence of specialized organelles such as mitochondria and chloroplasts more than a billion years ago.
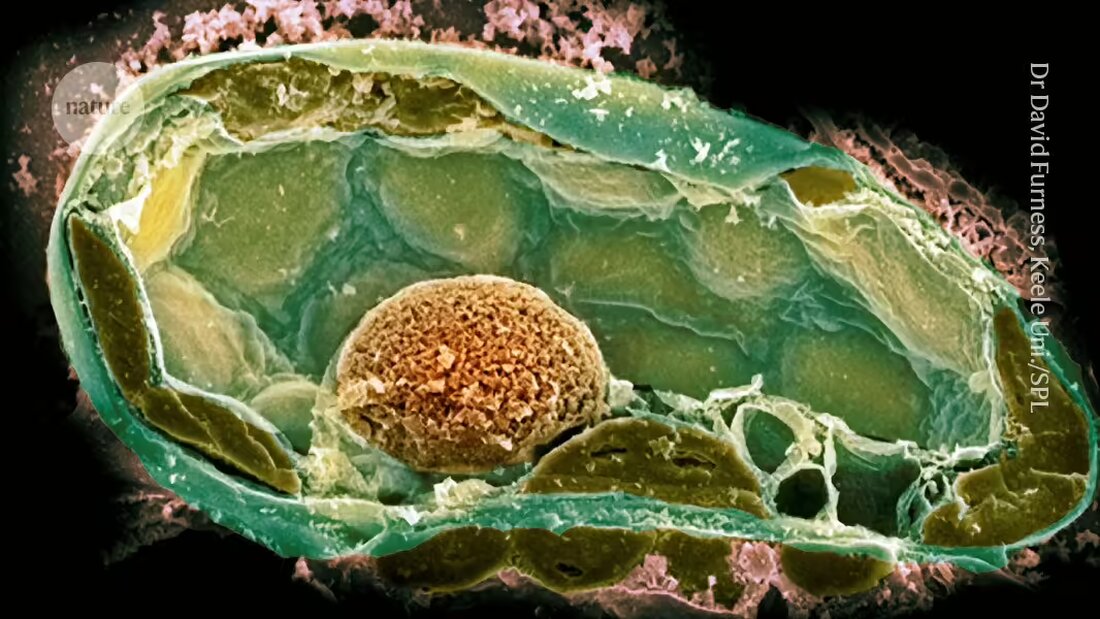
Endosymbiotic relationships, in which a microbacterial partner lives harmoniously within the cells of another organism, are found in numerous life forms, including insects and fungi. Scientists believe that mitochondria - the organelles responsible for energy production in cells - arose when a bacterium found refuge in an ancestor of eukaryotic cells. Chloroplasts emerged when an ancestor of plants absorbed a photosynthetic microorganism.
Determining the factors that formed and maintained these connections is difficult because they occurred so long ago. To get around this problem, a team led by microbiologist Julia Vorholt at the Swiss Federal Institute of Technology in Zurich (ETH Zurich) has developed endosymbiotic relationships in the laboratory in recent years. Their approach uses a 500-1000 nanometer wide needle to puncture host cells and then introduce bacterial cells one at a time.
However, the first attempts often failed; one reason for this was that the potential symbiote divided too quickly and killed its host 2. The team had more success when they recreated a natural symbiosis between some strains of the fungal plant pathogen Rhizopus microsporus and the bacterium Mycetohabitans rhizoxinica, which produces a toxin that protects the fungus from predation.
However, introducing bacterial cells into the fungi was challenging because they have thick cell walls that maintain high internal pressure. After piercing the wall with the needle, the researchers used a bicycle pump — later a compressor — to maintain enough pressure to introduce the bacteria.
After the initial shock of the “surgery,” the fungi continued their life cycles, producing spores, some of which contained bacteria. When these spores germinated, bacteria were also present in the cells of the next generation of fungi. This showed that the new endosymbiosis could be transferred to the offspring - a crucial finding.
However, the germination success of the bacteria-containing spores was low. In a mixed population of spores (some with bacteria and some without), those containing bacteria disappeared after two generations. To improve relationships, the researchers used a fluorescent cell sorter to select spores containing bacteria - which had been tagged with a glowing protein - and propagated only those spores in future rounds of reproduction. After ten generations, the spores containing bacteria germinated almost as efficiently as those without bacteria.
The basis for this adjustment is not clear. Genome sequencing identified some mutations associated with improved germination success in the fungus - a strain of R. microsporus not known to carry endosymbionts - and found no changes in the bacteria.
The line that germinated most efficiently appeared to limit the number of bacteria in each spore, says Gabriel Giger, co-author of the study and a microbiologist at ETH Zurich. "There are ways in which these two partners can live together better and more easily. That is something that is very important for us to understand."
Researchers don't yet know much about the immune system of fungi. But Thomas Richards, an evolutionary biologist at the University of Oxford, UK, wonders whether a fungal immune system prevents symbiosis - and whether mutations in this system could facilitate the relationships. “I’m a big fan of this work,” he adds.
Eva Nowack, a microbiologist at Heinrich Heine University Düsseldorf, Germany, was surprised at how quickly adaptations to symbiotic life seemed to emerge. In the future, she would like to see what happens after even longer periods of time; for example, after more than 1,000 generations.
The development of such symbioses could lead to the creation of new organisms with useful properties, such as the ability to consume carbon dioxide or atmospheric nitrogen, says Vorholt. “That’s the idea: to create new properties that an organism doesn’t have and that would otherwise be difficult to implement.”
-
Giger, G.H. et al. Nature https://doi.org/10.1038/s41586-024-08010-x (2024).
-
Gäbelein, C. G., Reiter, M. A., Ernst, C., Giger, G. H. & Vorholt, J. A. ACS Synth. Biol. 11, 3388–3396 (2022).

 Suche
Suche
 Mein Konto
Mein Konto
