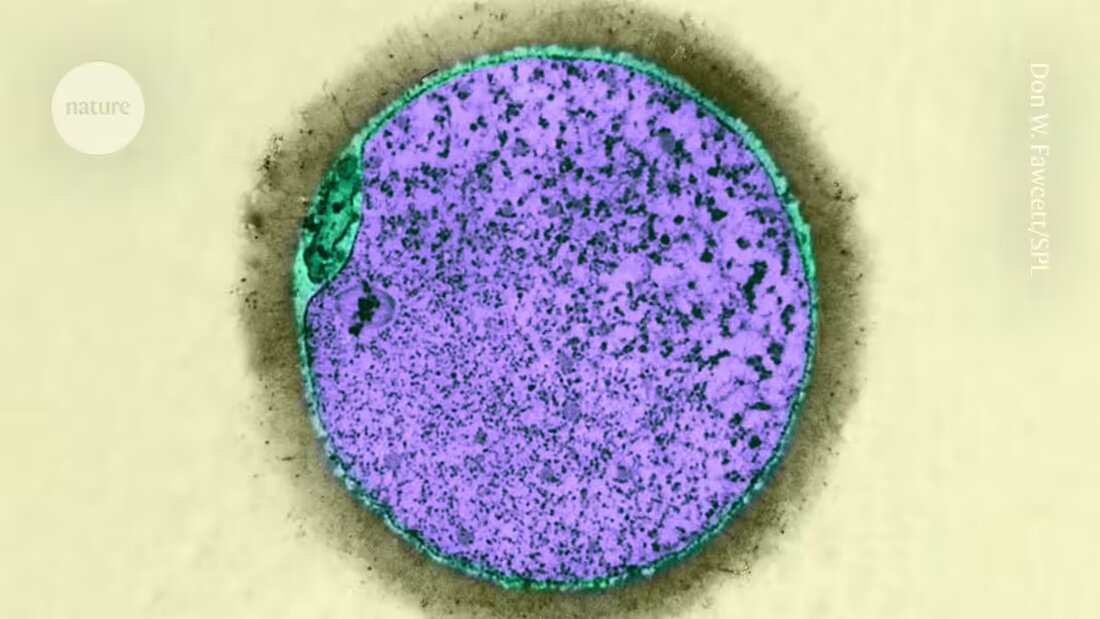
The growth of immature eggs from old mice in the ovarian structures of young mice can reverse signs of aging in the eggs 1.
“You can think of this as a five-star anti-aging spa for the old eggs,” says Rong Li, a cell biologist at the National University of Singapore (NUS), who contributed to a study describing the results.
When the rejuvenated eggs were fertilized, the resulting embryos were almost four times more likely to produce healthy offspring than eggs that had matured in an ancient environment. The results were announced todayNature agingpublished.
In the mammalian ovaries, structures known as follicles house a maturing egg cell called a Oocyte is referred to. After development, these are released as eggs ready for fertilization. However, with age it decreases Number and quality of oocytes. Researchers have investigated the role of oocyte aging in infertility 2studied, but Li and her colleague Wang HaiYang, also a cell biologist at NUS, focused on follicle aging.
The latest study provides "the first solid, strong evidence" that follicles can improve the quality of maturing eggs, says Suzannah Williams, an ovarian biologist at the University of Oxford, UK. They “did a fantastic job.”
Old to young
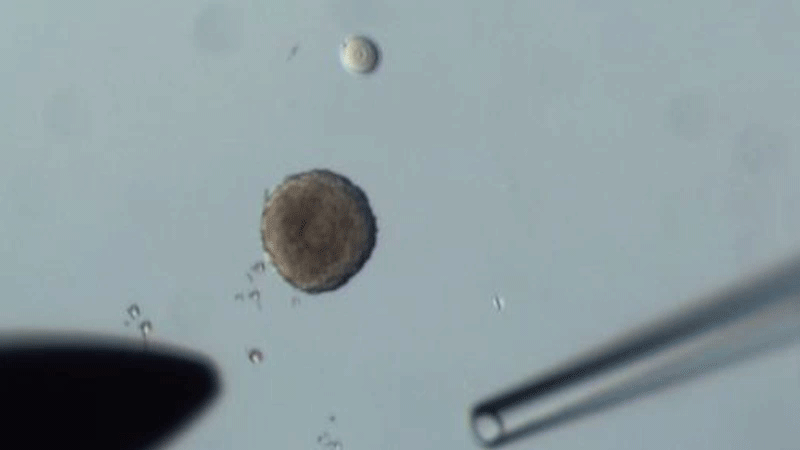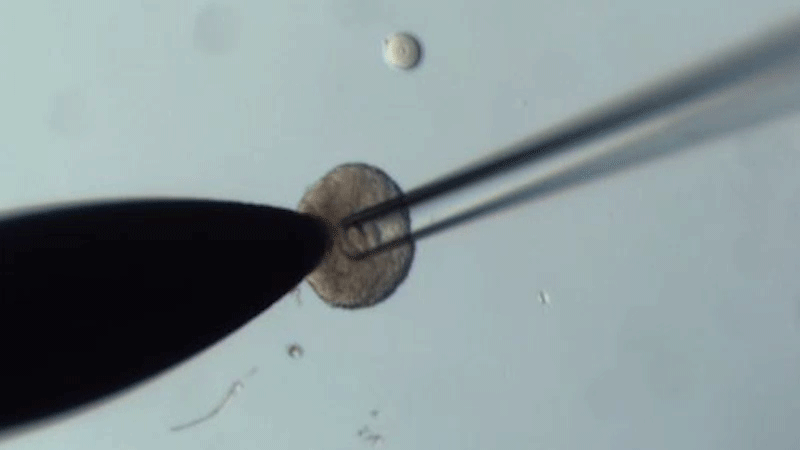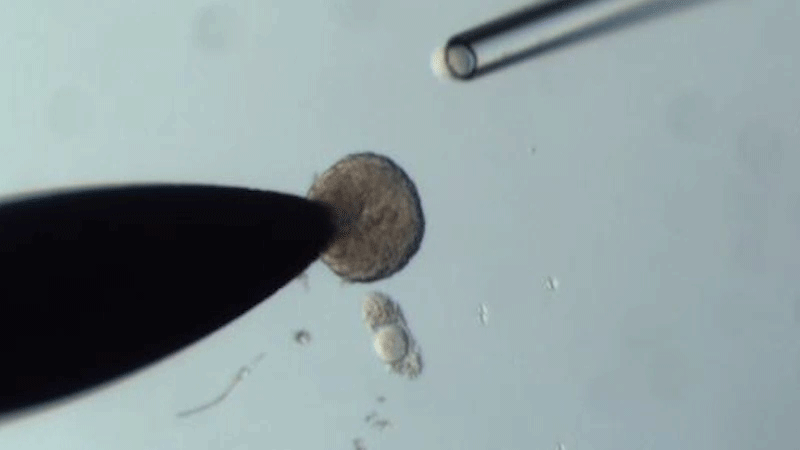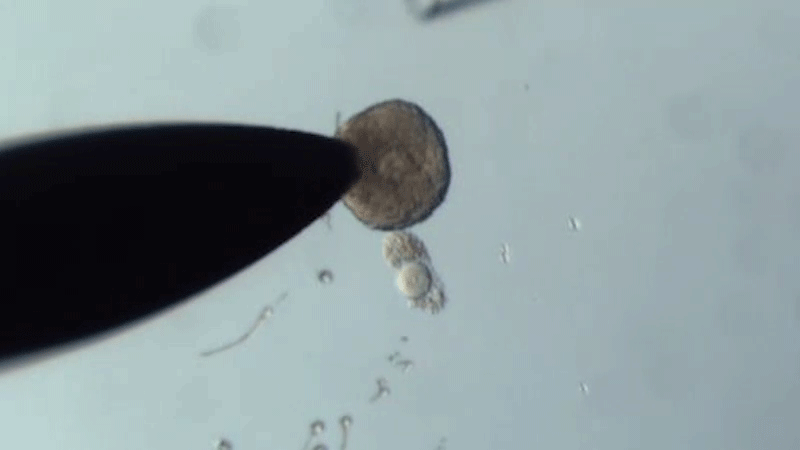
The researchers swapped oocytes from 14-month-old mice that were about to become infertile with those in the follicles of 2-month-old mice that were at their reproductive peak - and vice versa.
They found that the quality of old oocytes improved when grown in young follicles compared to those matured in old follicles. In particular, after treatment with young tissue, the oocytes had fewer chromosomal abnormalities, improved mitochondrial function, and their gene expression and metabolite production profiled more closely to the patterns of young oocytes.
Meanwhile, young oocytes grown in old follicles showed increased signs of aging.
The researchers fertilized the eggs and transferred them to surrogate mothers. Embryos derived from rejuvenated oocytes had significantly higher chances of producing an offspring than old oocytes grown in old follicles. Nevertheless, the success rate did not reach that of the offspring derived from young oocytes grown in young follicles.
The results suggest that oocyte aging is partially reversible and that the surrounding cellular environment plays an important role in the process, says Li.
More connections
The researchers took a closer look at what might drive oocyte rejuvenation. Tunnel-like structures connecting oocytes to follicles, known as transzonal projections (TZPs), transport important molecules to supply nutrients to the oocytes. The researchers found a higher density of TZPs in old oocytes encapsulated in young follicles compared to those encapsulated in old follicles.
If the results translate to humans, Li suggests it could lead to cell therapies that improve egg quality in older people.
However, Williams explains that this translation will be difficult because of the challenges of obtaining sufficient donor tissue and growing it in the laboratory for long enough. Still, she says, “It’s a proof of concept.”

 Suche
Suche
 Mein Konto
Mein Konto